🌑Knowledge Drop – 71: India’s Biosecurity in the Age of Biotechnology| For prelims: Highly expected MCQs; Booster Notes; Word-Flash Facts | For Mains: All G.S Papers: High Quality Essays ; Model Answers; Case Studies and beyond on iasmonk.com
🔗 This Knowledge Drop has a Mains-focused companion on iasmonk.com
“For answer-writing enrichment beyond Prelims, visit iasmonk.com.”

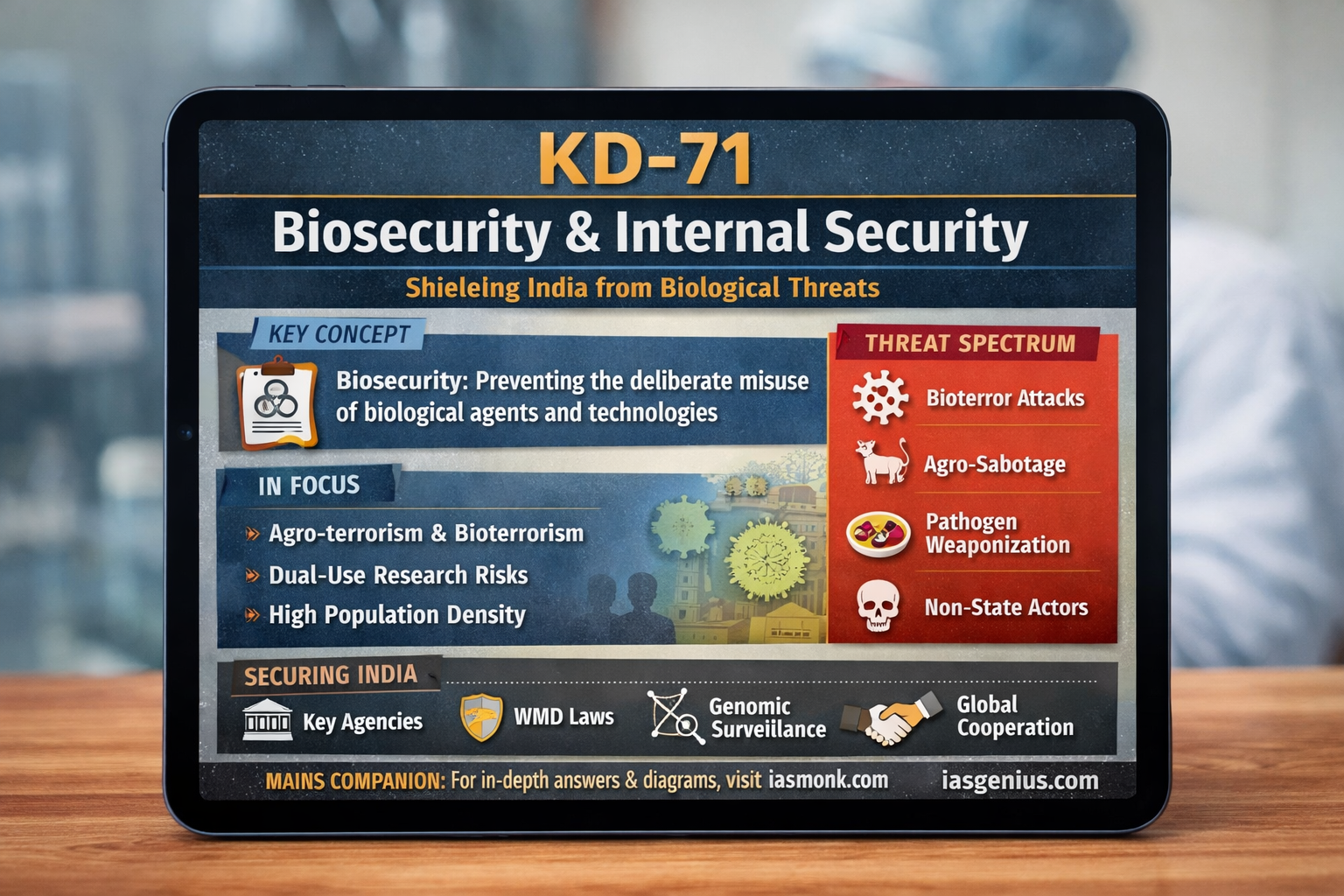
India’s Biosecurity in the Age of Biotechnology
Post Date: 24-12-2025
Syllabus: GS-3 | 🧬 Biotechnology | 🛡️ Internal Security
Context ⚠️
Rapid advances in biotechnology have increased the risk of deliberate misuse of biological agents by state and non-state actors. This has made the strengthening of India’s biosecurity framework a critical national security priority.
What is Biosecurity? 🧪
Biosecurity refers to the set of policies, practices, and institutional systems designed to prevent the intentional misuse of biological agents, toxins, or biotechnologies.
It includes:
- Securing laboratories handling dangerous pathogens
- Preventing unauthorised access to biological materials
- Detecting and responding to deliberate disease outbreaks
🔹 Biosecurity extends beyond human health to include animal and plant health.
Biosecurity vs Biosafety
- Biosafety focuses on preventing accidental release of pathogens
- Biosecurity focuses on preventing intentional misuse
➡️ A strong biosafety regime strengthens overall biosecurity.
Why Biosecurity Is Critical for India 🇮🇳
Demographic Vulnerability
- Large population and high density increase the potential impact of biological incidents
Agricultural Dependence 🌾
- Heavy reliance on agriculture and livestock
- Vulnerable to agro-terrorism and transboundary animal diseases
Biotechnology Expansion 🧬
- Rapid growth in biotech research
- Challenges in regulating dual-use research (civilian + military applications)
Non-State Actor Threats
- Growing interest in low-cost, high-impact biological agents
- Reports of alleged preparation of Ricin (derived from castor oil) for terror use underline emerging risks
India’s Existing Biosecurity Architecture 🏛️
Institutional Framework
- Department of Biotechnology — research governance and lab safety
- National Centre for Disease Control — outbreak surveillance and response
- Department of Animal Husbandry and Dairying — livestock biosecurity
- Plant Quarantine Organisation of India — agricultural biosecurity
- National Disaster Management Authority — biological disaster guidelines
Legal Instruments ⚖️
- Environment (Protection) Act, 1986 — hazardous microorganisms & GMOs
- Weapons of Mass Destruction and Their Delivery Systems Act, 2005 — criminalises biological weapons
- Biosafety Rules, 1989
- Recombinant DNA Research & Biocontainment Guidelines (2017)
International Measures 🌍
Biological Weapons Convention
- Prohibits development, production, acquisition, stockpiling and use of biological and toxin weapons
- Entered into force in 1975
- First treaty banning an entire category of WMDs
Australia Group
- Informal forum to prevent proliferation of chemical and biological weapons
- Harmonises export controls on dual-use materials and technologies
Global Best Practices 🌐
- United States: National Biodefense Strategy (2022–2028) integrating health, defence and biotech oversight
- China: Biosecurity Law (2021) treats biotechnology and genetic data as national security assets
- United Kingdom: Biological Security Strategy (2023) focuses on biosurveillance and rapid response
Way Ahead for India 🚀
- Establish a comprehensive national biosecurity framework with clear leadership
- Update laws to regulate dual-use research and synthetic biology
- Invest in:
- Genomic surveillance
- Microbial forensics
- Early-warning and biosurveillance systems
- Improve coordination between health, agriculture, defence and intelligence agencies
IAS Monk Whisper 🌀
In the age of biotechnology, security begins not at borders, but in laboratories, data and cells.
👉 KD-71 Prelims Booster Notes :
Upgrading India’s Biosecurity in the Age of Biotechnology
GS-3 | Biotechnology | Internal Security
Why in News?
Rapid advances in biotechnology have increased risks of deliberate misuse of biological agents, prompting calls to strengthen India’s biosecurity framework.
Biosecurity: Core Concept
- Biosecurity = policies, practices, and institutions to prevent intentional misuse of biological agents, toxins, and biotechnologies.
- Covers:
- Securing labs handling dangerous pathogens
- Preventing unauthorised access to biological materials
- Detecting/responding to deliberate disease outbreaks
- Extends beyond humans to animal and plant health.
Biosecurity vs Biosafety
- Biosafety: prevents accidental release of pathogens.
- Biosecurity: prevents intentional misuse.
➡️ Strong biosafety → stronger biosecurity.
Why Biosecurity Is Critical for India
- High population density → high impact of biological incidents
- Agriculture & livestock dependence → risk of agro-terrorism and transboundary animal diseases
- Biotech expansion → challenge of dual-use research (civilian + military)
- Non-state actors → low-cost, high-impact agents (e.g., reports of ricin preparation)
India’s Biosecurity Architecture
Institutions
- Department of Biotechnology — research governance, lab safety
- National Centre for Disease Control — surveillance & response
- Department of Animal Husbandry & Dairying — livestock biosecurity
- Plant Quarantine Organisation of India — agri imports/exports
- National Disaster Management Authority — biological disaster guidelines
Legal Instruments
- Environment (Protection) Act, 1986 — hazardous microbes & GMOs
- WMD Act, 2005 — criminalises biological weapons
- Biosafety Rules, 1989
- Recombinant DNA & Biocontainment Guidelines (2017)
International Measures
- Biological Weapons Convention (1975)
- Bans development, production, stockpiling, use of biological/toxin weapons
- First treaty to ban an entire WMD category
- Australia Group
- Harmonises export controls on dual-use materials/technologies
Global Best Practices (Quick Recall)
- USA: National Biodefense Strategy (2022–2028) — health-defence-biotech integration
- China: Biosecurity Law (2021) — biotech & genetic data as national security
- UK: Biological Security Strategy (2023) — biosurveillance & rapid response
Way Ahead (High-Yield)
- Create a comprehensive national biosecurity framework with clear leadership
- Update laws for dual-use research & synthetic biology
- Invest in:
- Genomic surveillance
- Microbial forensics
- Early-warning systems
- Improve coordination across health, agriculture, defence, intelligence
Prelims Traps ⚠️
- ❌ Biosecurity = biosafety
- ❌ Biosecurity limited to human health
- ❌ India lacks legal instruments on biological weapons
- ❌ Dual-use research is risk-free
One-Liners for UPSC
- Biosecurity addresses intent, biosafety addresses accident
- Biotechnology turns labs and data into security frontiers
- Agro-terrorism is a core biosecurity risk for India
IAS Monk Whisper 🌀
In the biotech era, national security is written in protocols, pathogens, and prevention.
Target IAS-2026+: Highly Expected Prelims MCQs :
📌 Prelims Practice MCQs
Topic: Upgrading India’s Biosecurity in the Age of Biotechnology
MCQ 1 | TYPE 1 — How Many Statements Are Correct?
Consider the following statements regarding biosecurity:
1)Biosecurity focuses on preventing the intentional misuse of biological agents and technologies.
2)Biosecurity is limited only to human health and disease control.
3)Safeguarding laboratories and preventing unauthorised access to pathogens are part of biosecurity.
4)Biosecurity and biosafety are identical concepts.
How many of the above statements are correct?
(a)Only one
(b)Only two
(c)Only three
(d)All four
🌀 Didn’t get it? Click here (▸) for the Correct Answer & Explanation.
🟩 Correct Answer: (b)Only two
🧠 Explanation:
1)✅True – Biosecurity addresses intentional misuse.
2)❌False – It also includes animal and plant health.
3)✅True – Lab security is a core component.
4)❌False – Biosafety deals with accidental release.
MCQ 2 | TYPE 2 — Two-Statement Type
Consider the following statements:
Statement I:A robust biosafety regime strengthens overall biosecurity.
Statement II:Biosafety focuses primarily on preventing deliberate biological attacks.
Which of the statements given above is/are correct?
(a)Only Statement I
(b)Only Statement II
(c)Both Statement I and II
(d)Neither Statement I nor II
🌀 Didn’t get it? Click here (▸) for the Correct Answer & Explanation.
🟩 Correct Answer: (a)Only Statement I
🧠 Explanation:
Statement I:✅True – Biosafety reduces risks that can be exploited deliberately.
Statement II:❌False – Biosafety focuses on accidental release, not deliberate attacks.
MCQ 3 | TYPE 3 — Code-Based Statement Selection
With reference to India’s biosecurity framework, consider the following statements:
1)The National Centre for Disease Control is responsible for outbreak surveillance and response.
2)The Plant Quarantine Organisation of India regulates agricultural imports and exports.
3)The Department of Biotechnology regulates livestock biosecurity and transboundary animal diseases.
Which of the statements given above is/are correct?
(a)1 and 2 only
(b)2 and 3 only
(c)1 only
(d)1,2 and 3
🌀 Didn’t get it? Click here (▸) for the Correct Answer & Explanation.
🟩 Correct Answer: (a)1 and 2 only
🧠 Explanation:
1)✅True – NCDC handles disease surveillance.
2)✅True – PQOI regulates agricultural biosecurity.
3)❌False – Livestock biosecurity is handled by the Department of Animal Husbandry and Dairying.
MCQ 4 | TYPE 4 — Direct Factual Question
Which one of the following international instruments was the first to ban an entire category of weapons of mass destruction?
(a)Chemical Weapons Convention
(b)Biological Weapons Convention
(c)Nuclear Non-Proliferation Treaty
(d)Australia Group
🌀 Didn’t get it? Click here (▸) for the Correct Answer & Explanation.
🟩 Correct Answer: (b)Biological Weapons Convention
🧠 Explanation:
The Biological Weapons Convention, which entered into force in 1975, was the first multilateral treaty to ban an entire class of WMDs.
MCQ 5 | TYPE 5 — UPSC 2025 Linkage Reasoning Format (I, II, III)
Consider the following statements:
Statement I:Strengthening biosecurity has become a critical internal security priority for India.
Statement II:Rapid advances in biotechnology have expanded the scope of dual-use research and materials.
Statement III:Non-state actors increasingly seek low-cost, high-impact biological agents for asymmetric attacks.
Which one of the following is correct?
A)Both Statements II and III are correct and both explain Statement I
B)Both Statements II and III are correct but only one explains Statement I
C)Only one of the Statements II and III is correct and that explains Statement I
D)Neither Statement II nor Statement III is correct
🌀 Didn’t get it? Click here (▸) for the Correct Answer & Explanation.
🟩 Correct Answer:A)Both Statements II and III are correct and both explain Statement I
🧠 Explanation:
Statement I reflects India’s rising biosecurity concern.
Statement II explains the expansion of risks due to dual-use biotechnology.
Statement III explains the security dimension posed by non-state actors.
Together, both statements logically explain the growing priority of biosecurity.
👉 KD-71 Mains Answer (250 words)
Upgrading India’s Biosecurity in the Age of Biotechnology
Rapid advances in biotechnology have transformed healthcare, agriculture and industry, but they have also amplified the risks of deliberate misuse of biological agents. In this context, strengthening biosecurity has emerged as a critical internal security priority for India.
Biosecurity refers to the policies, practices and institutional systems designed to prevent the intentional misuse of biological agents, toxins and biotechnologies. Unlike biosafety, which focuses on preventing accidental releases, biosecurity addresses malicious intent and therefore has direct national security implications. It extends beyond human health to include animal and plant health, making it vital for a country like India that is heavily dependent on agriculture and livestock.
India’s vulnerability arises from multiple factors. High population density magnifies the impact of biological incidents, while rapid expansion of biotechnology research increases challenges in regulating dual-use research with civilian and military applications. Further, non-state actors are increasingly attracted to low-cost, high-impact biological agents, as reflected in reports of attempted use of toxins such as ricin. Agro-terrorism and transboundary animal diseases pose additional risks.
India has developed a multi-layered biosecurity architecture involving the Department of Biotechnology, National Centre for Disease Control, Plant Quarantine Organisation and the National Disaster Management Authority, supported by legal instruments such as the Environment (Protection) Act, 1986 and the WMD Act, 2005. Internationally, India is committed to the Biological Weapons Convention, which bans biological and toxin weapons.
However, existing mechanisms remain fragmented. Going forward, India should establish a comprehensive national biosecurity framework with clear leadership, update regulations for synthetic biology and dual-use research, and invest in genomic surveillance, microbial forensics and early-warning systems. Enhanced coordination across health, agriculture, defence and intelligence agencies will be essential to secure India in the biotechnology era.
KD-71 | Diagram-Based Enrichment (Mains Value Add)
Theme: Biosecurity × Biotechnology × Internal Security
Diagram 1: Biosecurity vs Biosafety (Conceptual Clarity)
Biological Risk Management
|
┌────────────┴────────────┐
| |
Biosafety Biosecurity(Accidental Risk) (Intentional Risk)
| |
Lab accidents Terror / Sabotage
Unsafe handling Bioweapons
Poor containment Dual-use misuse
Use in answer:
“While biosafety addresses accidental release of pathogens, biosecurity focuses on preventing intentional misuse, giving it direct internal security relevance.”
Diagram 2: India’s Biosecurity Threat Spectrum
Biosecurity Threats
|┌───────────┬───────────┬───────────┐
| | | |
Human Agriculture Livestock Environment
Health (Agro- (Animal (Plant pests,
Bioterror terrorism) diseases) invasive species)
Use in answer:
“India’s biosecurity concerns span human, animal and plant health, making it a whole-of-economy security issue.”
Diagram 3: Dual-Use Biotechnology Risk Chain:
Biotech Research
↓
Dual-use Knowledge
↓
Legitimate Use ←→ Malicious Misuse
(Medicine, Agri) (Bioterror, toxins)
Use in answer:
“Rapid biotechnology expansion increases dual-use risks, requiring regulatory oversight without stifling innovation.”
Diagram 4: India’s Existing Biosecurity Architecture
National Biosecurity
|┌───────────┬───────────┬───────────┬───────────┐
| | | | |
DBT NCDC DAHD PQOI NDMA
(Labs & (Disease (Livestock (Plant (Biological
Research) Surveillance) Biosecurity) Quarantine) Disasters)
Use in answer:
“India has multiple agencies dealing with biosecurity, but coordination remains fragmented.”
Diagram 5: Way Forward — Integrated Biosecurity Framework
National Biosecurity Framework
|
┌───────────────┼───────────────┐
| | |Legal Reform Surveillance & Coordination
(Dual-use, Forensics (Health, Agri,
Synthetic Bio) (Genomics) Defence, Intelligence)
Use in answer:
“An integrated national biosecurity framework with legal clarity, surveillance capacity and inter-agency coordination is essential.”
How to Use in UPSC Answer (Smart Tip)
Write one linking line like:
“As illustrated in the diagram, India’s biosecurity challenge spans accidental, intentional and dual-use risks, requiring an integrated institutional response.”
This signals conceptual clarity + visual intelligence to the examiner.
For more details, visit: iasmonk.com
IAS Monk Whisper 🌀
A diagram in Mains is not art — it is compressed reasoning.
Reusable GS-3 Biosecurity Conclusion Paragraph — crisp, forward-looking, and examiner-friendly.
You can deploy this in any GS-3 answer touching biotechnology, health security, internal security, agriculture, or disaster management.
Reusable GS-3 Conclusion | Biosecurity
In the contemporary biotechnology era, biosecurity has evolved from a public health concern into a core dimension of national security. For India, safeguarding against biological threats requires moving beyond fragmented sectoral responses towards a comprehensive, coordinated and future-ready biosecurity framework. Strengthening legal oversight of dual-use research, investing in genomic surveillance and microbial forensics, and ensuring seamless coordination among health, agriculture, defence and intelligence agencies are critical. Equally important is balancing innovation with regulation so that scientific progress is not stifled while security risks are minimised. A resilient biosecurity architecture will not only protect India from deliberate biological threats but also enhance preparedness against natural outbreaks, agro-terrorism and emerging biotechnological risks, thereby reinforcing national resilience in an increasingly uncertain world.
Reusable GS-3 Intro Paragraph (Biosecurity) — sharp, conceptual, and UPSC-friendly.
You can open any GS-3 answer on biotechnology, internal security, health security, agro-terrorism, or disaster management with this.
Reusable GS-3 Introduction | Biosecurity
Rapid advances in biotechnology have expanded humanity’s capacity to prevent disease and enhance agricultural productivity, but they have simultaneously heightened the risks associated with the intentional misuse of biological agents and technologies. In this context, biosecurity has emerged as a critical intersection of biotechnology, public health and internal security. For a country like India, with high population density, deep dependence on agriculture and a rapidly expanding biotechnology sector, strengthening biosecurity is essential not only for safeguarding public health but also for protecting national security and economic stability.
Reusable GS-3 Keywords & Phrases Bank for Biosecurity Answers — examiner-friendly, high-scoring language you can sprinkle across introductions, bodies, diagrams and conclusions.
Use these selectively. One or two per answer is enough to lift quality.
Reusable GS-3 Keywords & Phrases | Biosecurity
Core Conceptual Keywords
- Biosecurity framework
- Intentional misuse of biological agents
- Dual-use biotechnology
- Biosafety–biosecurity continuum
- Biological threat spectrum
- Bio-risk governance
- Biological incident preparedness
Internal Security & Risk Language
- Non-state actor proliferation
- Low-cost, high-impact asymmetric threats
- Agro-terrorism vulnerabilities
- Transboundary animal diseases
- Deliberate disease outbreaks
- Bioterrorism risk landscape
Governance & Policy Vocabulary
- Whole-of-government approach
- Inter-agency coordination mechanisms
- Regulatory oversight of dual-use research
- Institutional fragmentation
- Legal harmonisation
- Transparent research governance
Science & Technology Terms
- Synthetic biology
- Genomic surveillance
- Microbial forensics
- Early-warning biosurveillance systems
- Pathogen containment protocols
- Biocontainment infrastructure
Health & Agriculture Linkages
- One Health approach (human–animal–environment)
- Food and nutritional security
- Livestock biosecurity
- Plant health surveillance
- Zoonotic spillover prevention
International & Strategic Phrases
- Compliance with international disarmament regimes
- Biological Weapons Convention obligations
- Export controls on dual-use materials
- Global biosecurity norms
- Cross-border disease intelligence sharing
Future-Oriented & Value-Add Phrases
- Security in the age of biotechnology
- Laboratories as security frontiers
- Prevention over reaction
- Innovation–regulation balance
- Resilient biosecurity architecture
One-Liner Insertions (Ready-to-Use)
- “Biosecurity converts laboratories and data into strategic security assets.”
- “Dual-use research blurs the line between scientific innovation and security risk.”
- “Agro-terrorism represents a silent but potent internal security threat.”
- “A robust biosecurity regime strengthens national resilience against both natural and deliberate biological events.”
IAS Monk Whisper 🌀
In GS-3 answers, the right keyword at the right place is worth a paragraph.
Reusable GS-3 Biosecurity Diagram Labels Bank — short, precise, and examiner-friendly, designed specifically for hand-drawn UPSC Mains diagrams.
You can mix and match these labels depending on the question.
Reusable GS-3 Biosecurity Diagram Labels
Core Diagram Titles
- Biosecurity Risk Framework
- Biosecurity vs Biosafety
- India’s Biosecurity Architecture
- Dual-Use Biotechnology Risk Pathway
- Integrated National Biosecurity Model
Risk & Threat Labels
- Intentional misuse
- Dual-use research
- Bioterrorism risk
- Agro-terrorism
- Non-state actors
- Transboundary diseases
Health–Agriculture–Security Link
- Human health
- Animal health
- Plant health
- One Health interface
- Zoonotic spillover
Institutional Blocks (India)
- DBT (Lab governance)
- NCDC (Surveillance)
- DAHD (Livestock biosecurity)
- PQOI (Plant quarantine)
- NDMA (Biological disasters)
Technology & Surveillance Labels
- Genomic surveillance
- Microbial forensics
- Biosurveillance systems
- Early-warning mechanisms
- Data integration
Governance & Legal Labels
- Regulatory oversight
- Dual-use controls
- Legal harmonisation
- Export controls
- Inter-agency coordination
International Dimension
- Biological Weapons Convention
- Export control regimes
- Global biosecurity norms
- International cooperation
Way-Forward Arrows (Use at Diagram Base)
- Integrated framework
- Capacity building
- Legal updates
- Technology investment
- Coordination & command
Exam-Smart Diagram Captions
- “Fragmented framework → Integrated response”
- “Prevention > Reaction”
- “Innovation with regulation”
- “Whole-of-government approach”
IAS Monk Whisper 🌀
A well-labelled diagram speaks faster than a paragraph.
Biosecurity | 20-Word Flash Facts (Prelims)
1)Biosecurity prevents intentional misuse of biological agents; biosafety prevents accidental release of pathogens.
2)Biosecurity covers human, animal, and plant health, not human health alone.
3)Dual-use biotechnology has civilian benefits but military or terror misuse potential.
4)Agro-terrorism targets crops and livestock to disrupt food security and economy.
5)High population density magnifies impact of biological incidents.
6)Non-state actors prefer low-cost, high-impact biological agents.
7)Ricin is a plant-derived toxin with potential misuse risk.
8)Genomic surveillance enables early detection of unusual or deliberate outbreaks.
9)Microbial forensics helps trace source and intent of biological incidents.
10)India’s biosecurity involves DBT, NCDC, DAHD, PQOI, NDMA.
11)Environment (Protection) Act, 1986 regulates hazardous microorganisms and GMOs.
12)WMD Act, 2005 criminalises biological weapons in India.
13)The Biological Weapons Convention bans biological and toxin weapons globally.
14)The Australia Group harmonises export controls on dual-use items.
15)Biosecurity strengthens prevention and preparedness in disaster management cycle.
16)Synthetic biology increases innovation speed and security risks simultaneously.
17)One Health approach links human–animal–environment biosecurity.
18)Fragmented institutions reduce biosecurity effectiveness; coordination is key.
19)Strong biosafety systems reinforce biosecurity outcomes.
20)Biosecurity today treats laboratories, data, and genomes as strategic assets.