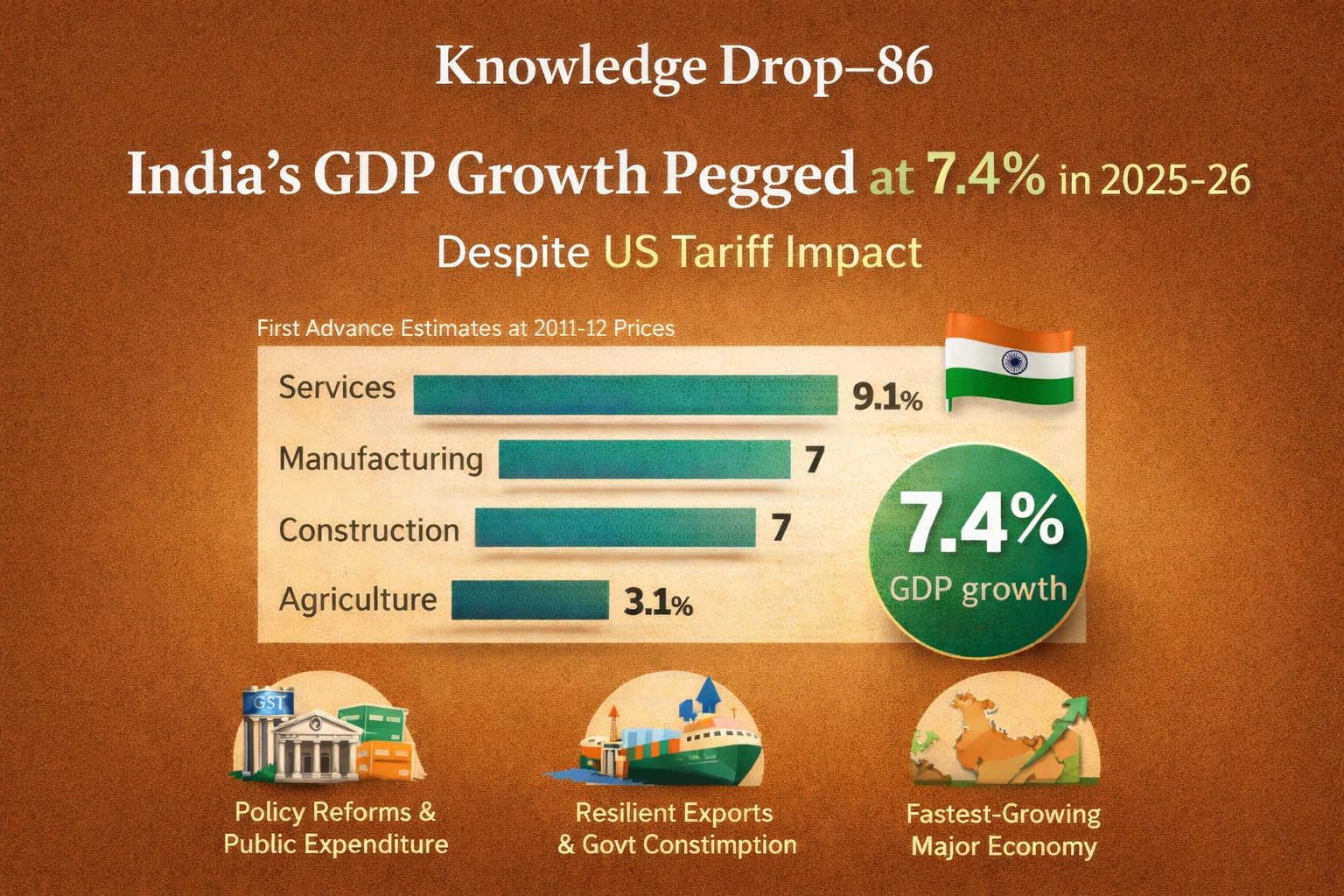
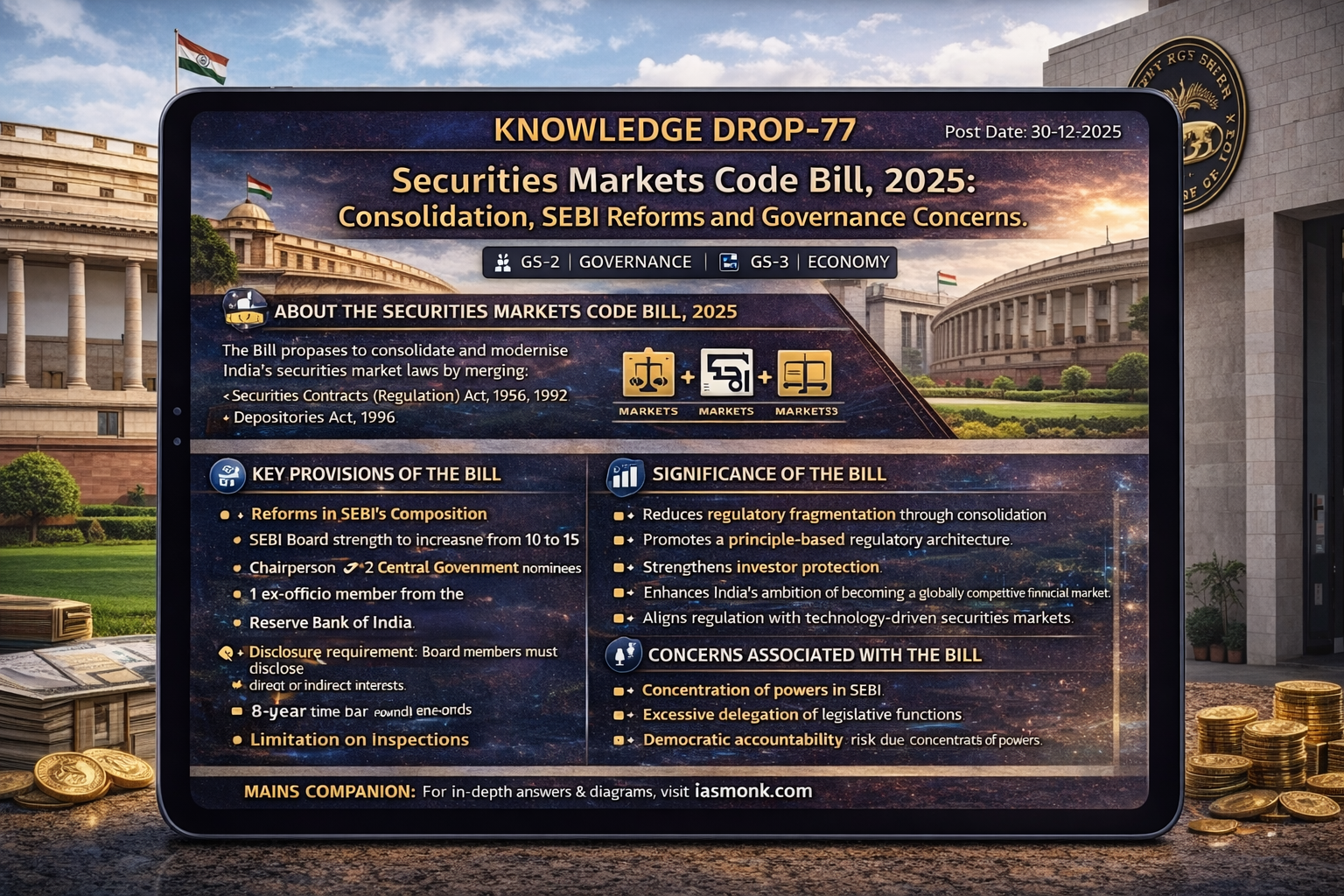
Question 31→ 2025 IAS Prelims GS I : Genius Classroom Explanation
📘 IAS Prelims 2025 — GS-I Q.31 | Classroom Explanation
(Environment & Ecology)
📌 Question
Q.31) Consider the following statements:
Statement I:
Studies indicate that carbon dioxide emissions from the cement industry account for more than 5% of global carbon emissions.
Statement II:
Silica-bearing clay is mixed with limestone while manufacturing cement.
Statement III:
Limestone is converted into lime during clinker production for cement manufacturing.
Which one of the following is correct in respect of the above statements?
(a) Both Statement II and Statement III are correct and both of them explain Statement I
(b) Both Statement II and Statement III are correct but only one of them explains Statement I
(c) Only one of the Statements II and III is correct and that explains Statement I
(d) Neither Statement II nor Statement III is correct
✅ Correct Answer: (a)
🧑🏫 Classroom Explanation
This question tests industrial sources of CO₂ emissions, especially the cement industry, a high-priority UPSC theme linking environment, industry, and climate change.
🔍 Key Concept: Why Cement Is Carbon-Intensive
The cement industry contributes well above 5% of global CO₂ emissions (≈ 7–8%), due to two fundamental reasons:
1️⃣ Chemical process emissions
2️⃣ Energy-intensive heating
🔍 Statement-wise Analysis
✅ Statement I: Correct
• Global studies (including IEA) show:
- Cement industry contributes ~7–8% of global anthropogenic CO₂ emissions
• This exceeds the 5% threshold mentioned in the statement
➡️ Statement I is correct.
✅ Statement II: Correct
• Cement manufacturing involves mixing:
- Limestone (CaCO₃)
- Silica-bearing clay
• Clay supplies:
- Silica (SiO₂)
- Alumina (Al₂O₃)
- Iron oxides
• The process requires very high temperatures (~1450°C):
- Generated using fossil fuels
- Leads to indirect CO₂ emissions from energy use
➡️ Statement II is correct and contributes to emissions.
✅ Statement III: Correct
• During clinker production (calcination):
CaCO₃ → CaO + CO₂
• Limestone is converted into lime (CaO)
• This chemical reaction:
- Releases process CO₂
- Accounts for ~50% of cement-sector emissions
➡️ Statement III is correct and directly explains Statement I.
🔗 Cause–Effect Link (Very Important)
• Statement I (high CO₂ emissions) is explained by:
- Statement II → energy-intensive heating
- Statement III → chemical release of CO₂
➡️ Both II and III explain I.
🧮 Logical Elimination (UPSC Style)
• Statement II → Correct
• Statement III → Correct
• Both explain Statement I
➡️ Correct option: (a)
🎯 Final Answer
✅ Correct Answer: (a)
📦 Info Box — Extra Points (Prelims Value Add)
• Cement emissions arise from:
- Process emissions (calcination)
- Energy emissions (kilns, fuel use)
• Globally, cement:
- Consumes 2–3% of total global energy
- Emits more CO₂ than aviation sector
• Climate mitigation strategies:
- Blended cement
- Alternative fuels
- Carbon capture and storage (CCS)
📍 GS Mapping
GS Paper III
Theme: Environment, Climate Change
Topic: Industrial CO₂ Emissions
Nature: Conceptual + Applied Science