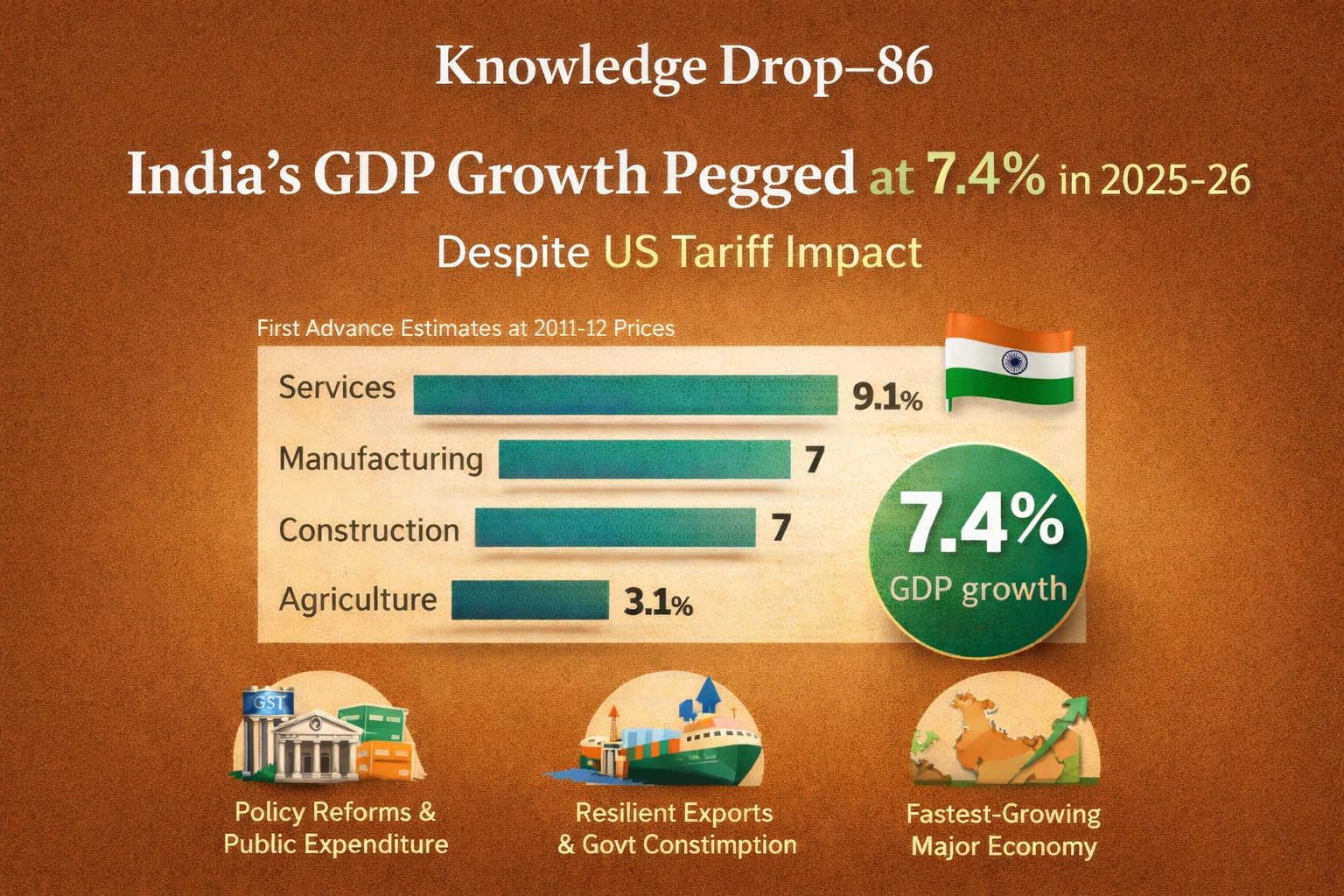
📘 Q.14 IAS Prelims 2022 — Environment & Ecology (Acid Rain)🧷 Authentic Classroom Explanation by IAS Monk
📌 The Question:
Consider the following:
- Carbon monoxide
- Nitrogen oxide
- Ozone
- Sulphur dioxide
Excess of which of the above in the environment is/are cause(s) of acid rain?
(a) 1, 2 and 3
(b) 2 and 4 only
(c) 4 only
(d) 1, 3 and 4
✅ Correct Answer: (b) 2 and 4 only
🧠 Classroom Explanation:
Acid rain is primarily caused by the atmospheric emissions of:
• Sulphur dioxide (SO₂)
• Nitrogen oxides (NOₓ)
These gases are released mainly from:
➡ Coal-based thermal power plants
➡ Petroleum refineries
➡ Vehicular exhausts
➡ Industrial combustion processes
Once in the atmosphere, SO₂ and NOₓ react with:
• Oxygen
• Water vapour
• Other oxidants
to form:
➡ Sulphuric acid (H₂SO₄)
➡ Nitric acid (HNO₃)
These acids dissolve in cloud droplets and fall to Earth as acid rain, snow, sleet, or dry deposition, damaging forests, crops, soils, freshwater ecosystems, and even monuments.
🔍 Why other options are incorrect:
• Carbon monoxide (CO) → Toxic, but does not form acids
• Ozone (O₃) → A secondary pollutant causing respiratory issues, not acid rain
🧠 Curiosity Raiser:
Did you know?
👉 The Taj Trapezium Zone (TTZ) was created mainly to protect the Taj Mahal from acid rain–induced marble corrosion.
📚 Enrich Notes (Prelims Essentials):
• Acid rain lowers soil pH and leaches nutrients like calcium & magnesium
• It releases toxic aluminium into soil and water bodies
• Major victims: coniferous forests, lakes, historical monuments
• International controls: Clean Air Act, UNECE protocols
Mnemonic:
👉 “Acid rain = SO₂ + NOₓ”
🪶 IAS Monk Whisper:
When invisible gases turn into falling acids, nature pays the bill.